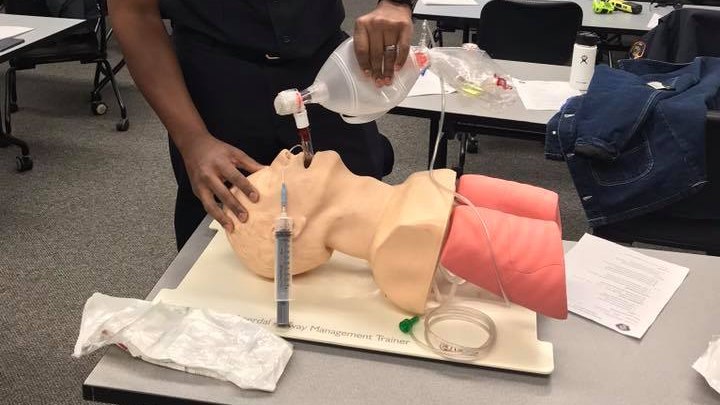
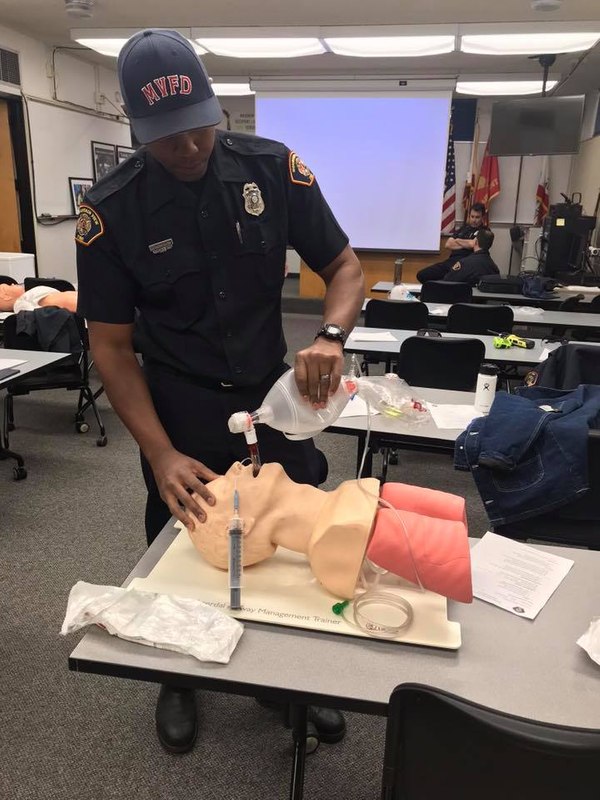
| Message: COVID-19 has changed some of the dynamics (and even statics) of how we do our job in EMS. The way we train, the PPE we don and the equipment that we use have all been impacted by this global pandemic.
If anything has been a positive from this virus, it's been our comprehensive understanding of our need to either thoroughly clean our equipment, or better transition toward disposable items to perform any number of our clinical functions ... especially airway management procedures.
I'm not talking about disposable versus reusable/durable endotracheal tubes and supraglottic airways (those have always been disposable within our industry). Rather, laryngoscope blades and continuous positive airway pressure (CPAP) devices come to mind as some of the airway management products that have seen their transition toward being disposable goods, rather than durable ones, even over the past five-plus years.
If anything has been a positive from this virus, it's been our comprehensive understanding of our need to either thoroughly clean our equipment, or better transition toward disposable items to perform any number of our clinical functions ... especially airway management procedures.
If anything has been a positive from this virus, it's been our comprehensive understanding of our need to either thoroughly clean our equipment, or better transition toward disposable items to perform any number of our clinical functions ... especially airway management procedures. (Photo/City of Mountain View Fire Department)
While disposable goods are more commonplace today, there are still opportunities for agencies to transition from one disposable product to another – or even opportunities to add disposable protective devices into your mix of durable goods – and there a small handful of questions that one should ask their vendor(s) when considering a move toward a new (or different) disposable airway management tool. Disposability doesn't necessarily mean that you need to sacrifice durability, but it does bring up a few considerations that single-use airway management tools might otherwise require.
Following are 6 questions to ask vendors before purchasing disposable airway management tools.
1. SAMPLES AND DEMOS
Before rolling a new product into the mix – or making a complete product switch – it's important to get some hands-on experience and perspective with whatever product you're looking to bring into your agency. Let your crews get their hands on a trial or demo, talk about its strengths and weaknesses, and even give them a chance to trial (break) it ... in a controlled environment, of course.
2. UP-FRONT COSTS
A complete changeover from machine-driven CPAP equipment toward completely disposable options will require some up-front costs in order to stock each of your ambulances and supply cabinets. Especially when this is a new product for your agency, now may be the optimal time to capitalize on bulk pricing in order to outfit (or re-fit) your agency and its fleet.
3. MULTIPLE (NEW) SIZES
Large is the new medium – just like how 40 is the new 30 – with some products, at least. Before you make a product switch, take a look at what the product's sizing options are and how they'll impact both your agency and your population base.
4. MAINTENANCE COSTS
Not so much in the sense of fixing items (these products are disposable, after all) – but, more in the sense of product expiration dates and replacement costs. Factor in your product use – over time – to get a bigger picture on the long-term impact that your new tools will cost.
5. COINCIDING PRODUCTS
Will transitioning to a new CPAP device require a new form of nebulizer for your agency, too? How about those disposable laryngoscope blades? Do they properly fit and work with your current laryngoscope handles? Does this new endotracheal tube come with a pre-loaded stylet, even if you're using a bougie for your intubations? Will changing one aspect of your supply chain affect another – for better, worse or indifferently?
6. PRODUCT TRAINING
Many disposable airway management devices are pretty straight forward and can be implemented into your system without hardly a hiccup. Others, on the other hand, may necessitate a need for a brief in-service training session, or even a skills competency review before they're phased in. Does the vendor already have training tutorials, videos and hand-outs available, or will your agency have to learn with more of an on-the-job approach?
TRANSITIONING TO A NEW PRODUCT
Change: this can be a cautionary or even dangerous word for some, even if it's just for a new brand of endotracheal tubes. How a product wrapper looks, its storage size or even a new stocking quantity all require communication; something that a successful transition or phase-in period absolutely needs. Communicate with your vendor about the potential benefits and disseminate this information to your providers.
About the author
Tim Nowak, AAS, BS, NRP, CCEMTP, SPO, MPO, CADS, is the founder & CEO of Emergency Medical Solutions, LLC, an EMS training and consulting company that he developed in 2010. Through this venture, he is the editor-in-chief of "EMS Director" magazine, a webinar/app-based continued education content developer, a columnist and blog writer, a product developer, an instructor and speaker, a podcast guest and host, and a social media influencer on LinkedIn.
Tim is also the assistant chief of Special Operations with a county-wide EMS agency based in Florida, where he oversees the planning and logistics sections, special operations functions and community paramedicine programs for the agency.
Tags Airway Management Budget Coronavirus (COVID-19) EMS Management Equipment Infectious Diseases Paramedic Chief |


Δεν υπάρχουν σχόλια:
Δημοσίευση σχολίου
Medicine by Alexandros G. Sfakianakis,Anapafseos 5 Agios Nikolaos 72100 Crete Greece,00302841026182,00306932607174,alsfakia@gmail.com,